Iodine business
What is iodine?
Iodine is a halogen element. Our corporate group produces iodine from the groundwater (brine water) pumped up at the time of producing natural gas, as the second business following the natural gas business. The purposes of use of iodine are raw materials for medicated gargle, X-ray contrast media, disinfectants, and fungicides. It is also used in the agricultural field, and has been recently utilized in the hi-tech field, including polarizing films for liquid crystal. However, unfortunately, Japan exports iodine as a raw material and imports processed products with added value in most cases. To improve this situation, “Forum on Iodine Utilization (FIU)” was established through the cooperation among industry, government, and academia in June 1998, for the purpose of promoting the research into iodine utilization and nurturing industries using iodine. It was renamed the Society of Iodine Science (SIS) in July 2007, and vigorously engages in activities, including the holding of international symposiums. Our corporate group, too, proactively participates in its activities, and pursues the new possibilities of iodine.
iodine

Merchandized iodine

Iodine essential for health

Iodine is a halogen element included in brine water, which accompanies natural gas, and an essential element for the human body, because it has vital physiological effects for our survival and growth. Japan is an insular country surrounded by the sea, so Japanese people can take a necessary amount of iodine from seaweed and other seafood. Meanwhile, some people are suffering from iodine deficiency disorder, such as underdevelopment, in landlocked countries, etc. where the intake of seafood is insufficient. Our company produces iodine as the second pillar of our business and exports most of it. Iodine is one of valuable domestic resources resource-poor Japan can export, and Japan (mostly Chiba Prefecture) is a main producer in the world, like Chile in South America.
History of iodineHISTORY OF IODINE
In 1811, Bernard Courtois in France discovered a phenomenon in which the purple steam with a pungent odor generated during the process of producing niter from seaweed ash becomes a black-purple scale-like material with metallic luster when it cools down. He entrusted a friend of his with the research into that material, and the friend announced the results of the research in 1813. This is the start of history of iodine.
In the following year 1814, Joseph Louis Gay-Lussac in France found that the material is an element that has the same properties as chlorine, and the material was named “iode” after “iodes,” which means purple in Greek.
That year, industrial manufacturing began, and in 1816, it was reportedly used as a sterilizing agent for pharmaceutical products. Then, it has been used broadly in not only the pharmaceutical field, but also the industrial field.
It is 1887 when iodine production was commercialized in a full-scale basis in Japan. Then, the iodine business thrived temporarily mainly in Chiba, thanks to the special demand during the Japanese-Sino War (1894-1895), the Japanese-Russo War (1904-1905), and the First World War (1914-1918). However, it declined due to the Showa Depression, which began in 1930, and the Pacific War, which broke out in 1941. Then, the iodine business started thriving again due to the special demand during the Korean War, which broke out in 1950, and the iodine in Chiba Prefecture was basked in the limelight as an export product, amid the excessive demand in the world. Our company started the iodine business via a subsidiary in 1937, and the iodine business became full-fledged in 1969 after trial & error and twists & turns. We have secured our position as one of the largest producers of iodine.
Purposes of use of iodine
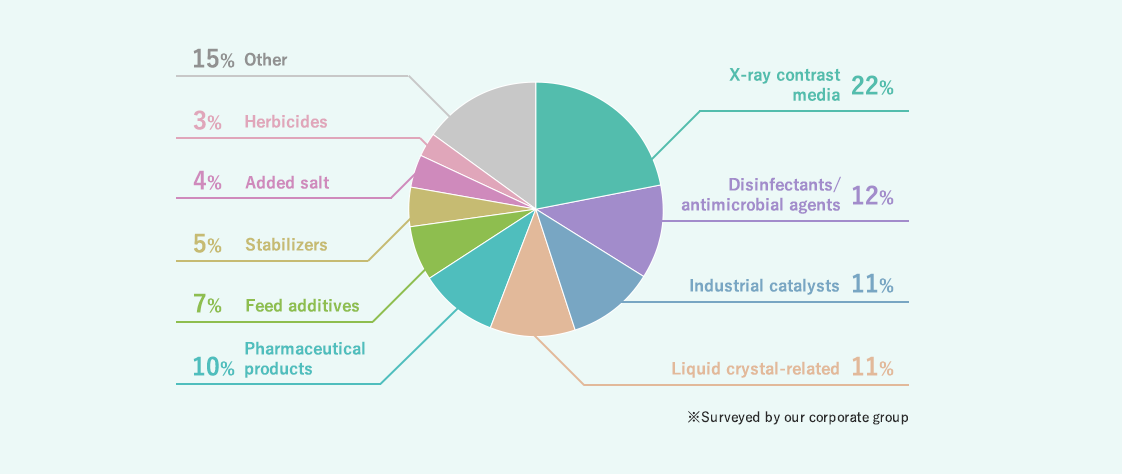
Composition of purposes of use of iodine
Iodine is used as a raw material for medicated gargle, X-ray contrast media, disinfectants, antimicrobial agents, etc. in our daily lives, and also for industrial catalysts and agriculture. Recently, it has been utilized in the hi-tech field, including the polarizing films of liquid crystal panels.
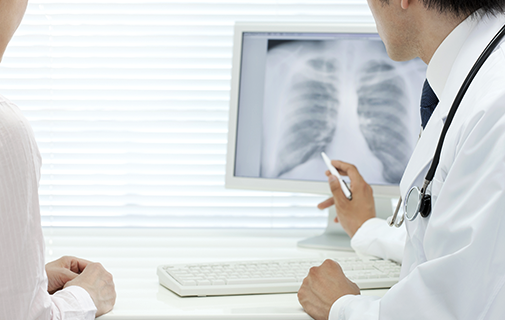
X-ray contrast media
Iodine can absorb X-rays considerably. By utilizing this property, we use iodine as an X-ray contrast agent at the time of medical diagnosis for blood vessels and organs.

Disinfectants and antimicrobial agents
Iodine is used for disinfectants and antimicrobial agents, by utilizing the intrinsic antimicrobial property of iodine. In general, it is well known that iodine is used for medicated gargle, antiseptics, etc.

Liquid crystal-related
Iodine is used for the polarizing films of liquid crystal panels, which are mounted on a wide array of products, such as TV sets, PCs, and cellphones. Polarizing films are indispensable for liquid crystal panels, so its market is expanding.

Feed additives and added salt
Iodine is essential for the survival and growth of human beings and animals. If the intake of iodine is insufficient, iodine deficiency disorder occurs, leading to dysfunction, such as underdevelopment. In order to prevent such diseases, iodine is blended in feed and salt.
Iodine, one of valuable domestic resources
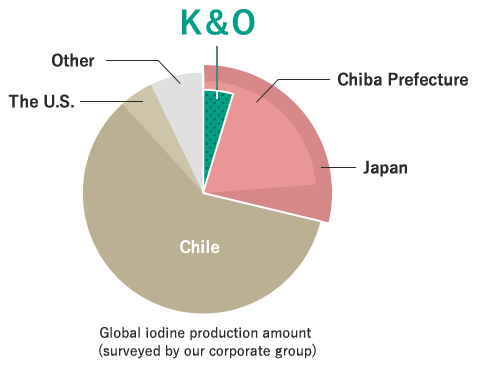
Japan produces the second largest amount of iodine after Chile in South America, accounting for about 30% of the global production amount. Japan is said to be resource-poor, but in terms of iodine, Japan is resource-rich.
About 80% of the total production amount in Japan is from Chiba Prefecture. Our corporate group produces about 15% of the production amount in Japan and about 5% of the global production amount. The iodine extracted in Chiba Prefecture is exported to the world as a raw material for various products, so it is one of resources Japan can boast to the world.
We produce about5% of the global iodine production amount.
We boast one of the largest production/sales amounts in the world.
Where does iodine exist?
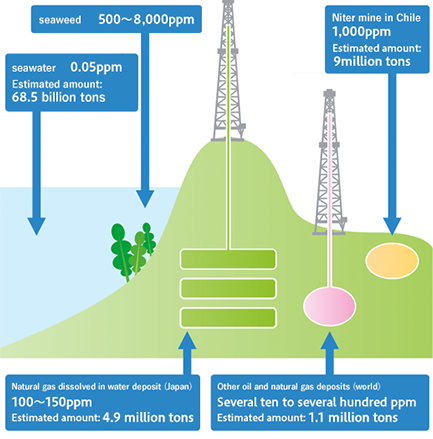
Iodine is distributed in seawater and soil, but iodine concentrations in seawater and soil are so low that it is difficult to earn profit from them. In the past, iodine was extracted from seaweed, in which iodine is concentrated, but at the present, iodine is extracted in limited areas, such as some oil fields and gas fields, where brine water upwells, and Chile in South America, where niter is mined. The brine water in Minami-Kanto Gas Field in Chiba Prefecture, where Japanese iodine makers, including our corporate group, produce iodine, includes 0.1 g/kg (100 ppm) of iodine, and the reserves of the oil field account for about 30% of the global reserves, making it one of the largest deposits in the world.
Raw materials and properties
The natural gas produced by our corporate group can dissolve in water, so it is pumped up with groundwater (brine water). The salt concentration of brine water is nearly equal to that of seawater, but the iodine concentration of brine water is nearly 2,000 times that of seawater, while the sulfuric acid concentration of brine water is very low.
Iodine reacts with starch, to become purple. It is a well-known element as a nutrient included in seaweed, such as kelp. Iodine as a product is solid and heavy like metal, and has black-purple luster. When heated, it liquefies at a relatively low temperature, and it easily sublimes like dry ice at room temperature. It also has a peculiar odor. Since it has strong oxidation power, it is processed into many iodine compounds to be used in various fields.
Comparison in components between brine water and seawater
| Item | Brine water [mg/l] | Seawater [mg/l] |
|---|---|---|
| Iodide ion (l-) | 110~130 | 0.05 |
| Chloride ion (Cl-) | 18,000〜19,500 | 18,230 |
| Bromide ion (Br-) | 120 | 56.2 |
| Sodium ion (Na+) | 10,000 | 9,350 |
| Potassium ion (K+) | 300 | 356 |
| Calcium ion (Ca2+) | 190 | 372 |
| Magnesium ion (Mg2+) | 500 | 1,160 |
| Sulfate ion (SO42-) | 0 | 2,450 |
| Bicarbonate ion (HCO3-) | 1,000 | 105 |
| Carbonate ion (CO32-) | - | 5.9 |
| Free carbon dioxide (Free CO2) | 10~30 | - |
| Ammonium ion (NH4+) | 120 | 1.5 |
| Borate ion (HBO32-) | 10 | 21.9 |
| Total iron (Total Fe) | 2~5 | 0.2 |
| pH | 7.9 | 8.2 |
|---|
References: Journal of Japanese Association for Petroleum Technology

| Chemical symbol | l | Specific gravity | 4.93 |
|---|---|---|---|
| Atomic number | 53(Halogen family) | Melting point | 113.7℃ |
| Atomic weight | 126.9 | Boiling point | 184.5℃ |
How to produce iodine
Two methods for producing iodine
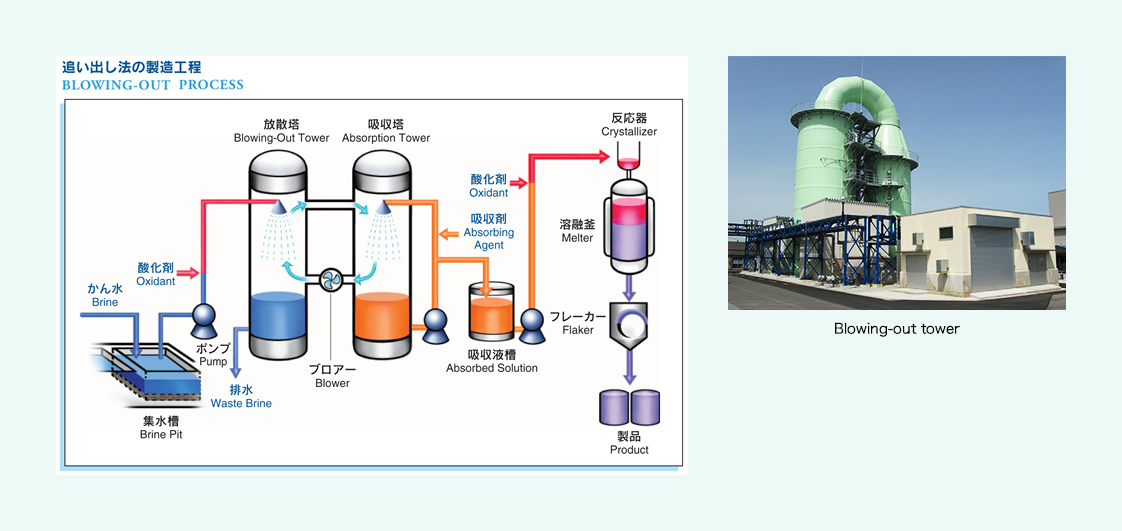
Blowing-out method
This method takes advantage of iodine’s property of vaporizing easily, and is suited for brine water treatment at high temperatures. Firstly, sand and impurities in brine water are removed through sedimentation, and an oxidant is added to transform iodine ions into iodine molecules. Next, this brine water is sprayed from the upper part of the blowing-out tower, so that the iodine in the brine water will vaporize in air. Then, this air is sucked toward the absorption tower, where an absorbing agent absorbs and concentrates iodine. Lastly, chlorine is added to the iodine-absorbed solution, to deposit crystals, and they are once dissolved before purification, and then our products are completed.
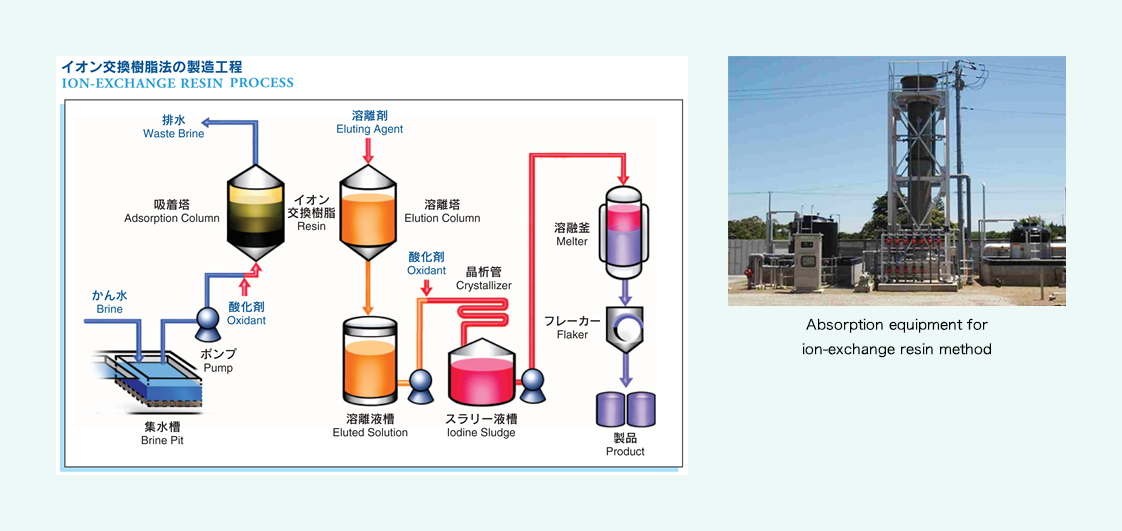
Ion-exchange resin method
Sand and impurities are removed from brine water through sedimentation and filtration, and an oxidant is added to transform iodine ions into iodine molecules. Next, this brine water is fed into the absorption column filled with ion-exchange resin, to absorb iodine. The resin that has absorbed iodine sufficiently is taken and put into the elution column, to elute iodine with sulfurous acid solution, and chlorine is added to the eluted solution, to deposit iodine crystals. They are once dissolved before purification, and then our products are completed.